
We wish we had saved one of the first edition videos, for the sake of comparison. Now the videos stream and the CD seems no longer on offer. The animations in the original were huge for the time (hydrogen was about 12 megabytes), so downloading and saving them was a wise use of bandwidth-or one could buy a CD for $37 (20 pounds).
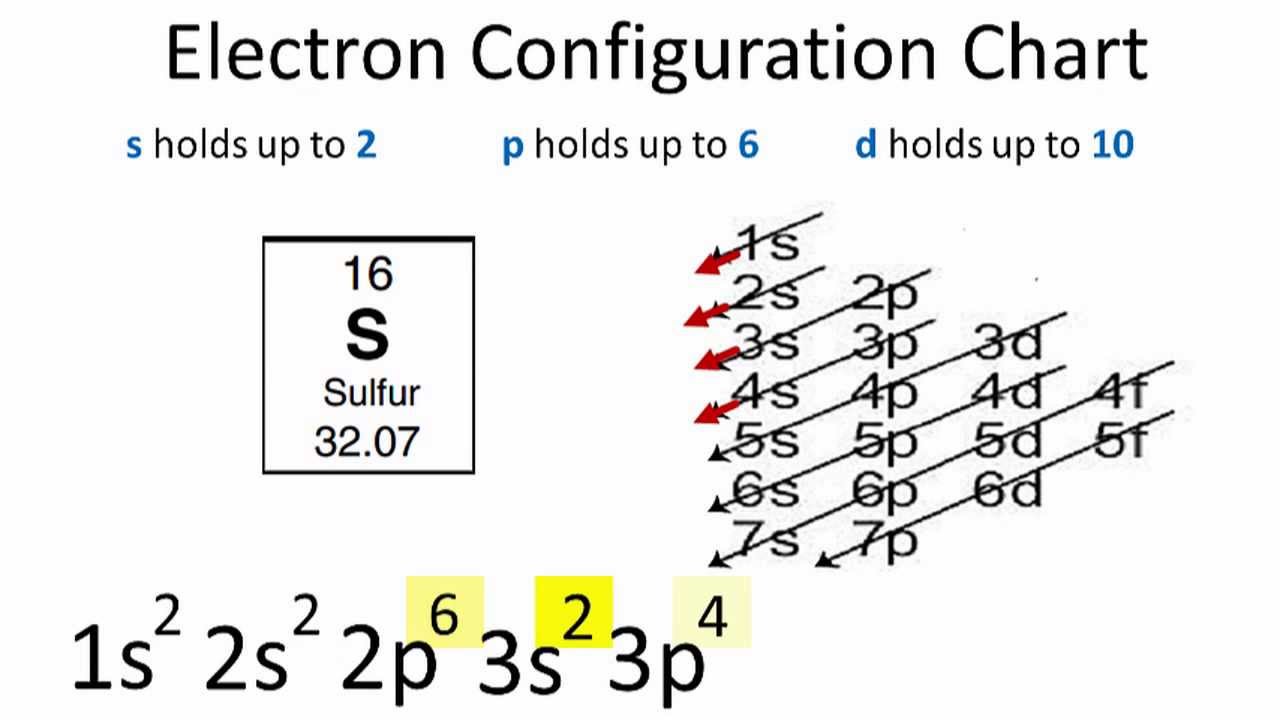
The current version is at least the second.

Society of Chemistry (UK) are truly exceptional. These values were determined using several different methods. resourcesīoth the content and design of this site created by the Royal Atomic radius, non-bonded Half of the distance between two unbonded atoms of the same element when the electrostatic forces are balanced. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides.Īcta Crystallographica, Section A. The coordination properties and ionic radius of actinium: A 120-year-old enigma.Ĭoordination Chemistry Reviews, vol. Gauthier J.-P.Deblonde, Mavrik Zavarin, and Annie B. From this pattern scientist are able to distance ebetween the atoms and from thst, Elementġ. I one shines x-rays through the crystel onto a sensor, such as a sheet of photographic film, a pattern of spots. Using only the periodic table arrange the following elements in order of increasing atomic radius: selenium, polonium, tellurium, sulfur. The atoms in a crystel are arannged in a regular pattern.
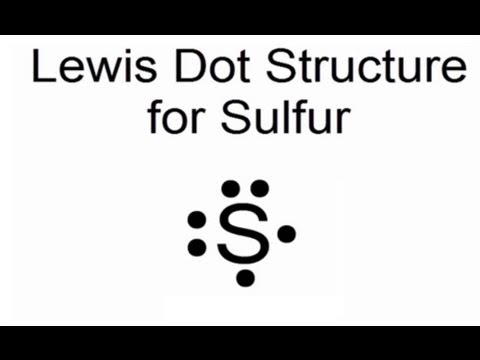
They are cfound in clouds.ĭistance beteen teh central charge and the outermost electron. larger because the n quantum number increases. n atom is not like a minature solre sysem either, though it is sometimes helpful to think of it that wat.An atoms electrons don't travil in regualr orbits. Compared with the atomic radius of oxygen (Z 8), the atomic radius of sulfur (Z 16) is. We know it has a posoitively charged center (the nucleus), which is surounded by mving electrosn. What does it mean to measure the “size” of an atom?Īn atom is not like a billiard ball. See more ideas about sulphur, electron configuration, atom project.


 0 kommentar(er)
0 kommentar(er)
